Research & Development |
D1
|
COVID-19 Research being carried out at the RUH
The World Health Organisation describes Coronavirus disease (COVID-19) as 'an infectious disease caused by a newly discovered coronavirus'.
The best way to prevent and slow down transmission of this new disease is be well informed about the COVID-19 virus, the disease it causes and how it spreads.
At this time, there are no specific vaccines or treatments for COVID-19. However, there are many ongoing clinical trials evaluating potential treatments'.
The Chief Medical Officer Chris Whitty has highlighted the importance of research in many of the Governments briefings and has contacted all Trusts in the UK to encourage participation in COVID-19 research.
The RUH is proud to be participating in a number of ethically approved research studies to help discover more about the disease and if there are treatments to help those affected.
Watch the film
COVID-19 antibody research - SIREN
First report from Public Health England's SIREN study, which is being carried out at the RUH, finds antibodies from past COVID-19 infection provide 83% protection against reinfection for at least 5 months (Jan 2021)- Reinfections in people with antibodies were rare – experts identified 44 potential reinfections among 6,614 participants who showed evidence of previous infection
- However, early evidence also suggests a small number of people with antibodies may still be able to carry and transmit COVID-19
- Public Health England continues to stress the importance of following the stay at home rules and remembering hands, face, space - whether you have had the virus or not
Background information
- PHE's SIREN (SARS-CoV-2 Immunity and Reinfection EvaluatioN) study has performed regular antibody and PCR testing on 20,787 healthcare workers, including frontline clinical staff and those in non-clinical roles, from 102 NHS trusts since the study commenced in June. 6,614 of these participants tested positive for COVID-19 antibodies upon recruitment.
- Of the 44 potential reinfections identified by the study, 2 were designated 'probable' and 42 'possible', based on the amount of confirmatory evidence available. If all 44 cases were confirmed, it would represent an 83% rate of protection from reinfection, while if only the 2 'probable' reinfections were confirmed, the rate would be 99%. Further research is ongoing to clarify this range.
- The study found that antibody protection after infection lasts for at least 5 months, on average, and scientists are currently studying whether protection may last for longer. This means that many people who contracted the disease in the first wave may now be vulnerable to catching it again.
- Both of the 2 'probable' reinfections reported having experienced COVID-19 symptoms during the first wave of the pandemic, but were not tested at the time. Both patients reported that their symptoms were less severe the second time. None of the 44 potential reinfection cases were PCR tested during the first wave, but all tested positive for COVID-19 antibodies at the point of recruitment to the study.
- This analysis occurred prior to the widespread dissemination of the new variant VOC202012/01, further work is underway in the laboratory to understand whether and to what extent antibodies also provide protection from this variant and future analysis will assess the impact of VOC202012/01 on symptomatic and asymptomatic infections in healthcare workers.
- The study will continue to follow participants for 12 months to explore how long any immunity may last, the effectiveness of vaccines and to what extent people with immunity are able to carry and transmit the virus.
Professor Susan Hopkins, Senior Medical Advisor at Public Health England and the SIREN study lead said:
"This study has given us the clearest picture to date of the nature of antibody protection against COVID-19 but it is critical people do not misunderstand these early findings".
"We now know that most of those who have had the virus, and developed antibodies, are protected from reinfection, but this is not total and we do not yet know how long protection lasts. Crucially, we believe people may still be able to pass the virus on".
"This means even if you believe you already had the disease and are protected, you can be reassured it is highly unlikely you will develop severe infections but there is still a risk that you could acquire an infection and transmit to others. Now more than ever it is vital we all stay at home to protect our health service and save lives".
"We are immensely grateful to our colleagues in the NHS for giving up their time to volunteer, and whose continued participation at a time of great stress is making this research possible."
The results of the study can be found here:
Current COVID-19 Studies at the RUH

The International Severe Acute Respiratory and emerging Infection Consortium has developed a for any severe or potentially severe acute infection of public health interest. This is a 'sleeping' study here at the RUH and is activated in exceptional circumstances such as we are currently experiencing.
The research teams from many specialties here at the RUH are collecting a large volume of data from all confirmed cases of COVID-19, this is anonymously and securely fed back to the ISARIC team so they can try to find better ways to manage and treat this infection in the future.
The data collected for this study is informing COVID-19 policy decisions at the highest level. The Chief Medical Officer and Department of Health and Social Care has asked that the data collection process continues as a priority exercise and the RUH research teams are continuing to collect data throughout this outbreak.

The RECOVERY Trial aims to identify treatments that may be beneficial for adults hospitalised with confirmed COVID-19.
This is a Randomised Control Trial (RCT), once a participant has been consented in to the study they allocated at random to one of the treatment arms. Here at the RUH the treatment arms available for randomisation are:
- Lopinavir-Ritonavir (commonly used to treat HIV)
- Low-dose Dexamethasone (a type of steroid, which is used in a range of conditions typically to reduce inflammation)
- Hydroxychloroquine (related to an anti-malarial drug)
- Azithromycin (an antibiotic)
The Recovery team will constantly review the information that is being fed back to them so that any effective treatment can be identified quickly and made available to patients.

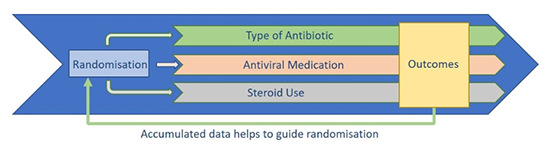
A Randomised, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired Pneumonia.
This is a large global trial, the aim of the study is to investigate which treatment options are best for patients admitted to ICU with severe pneumonia. This study is an 'adaptive' study. This means that the chances of being assigned to any of the treatment options may change on the basis of the study results, in favour of the most promising treatment.
Genetics of Mortality in Critical Care
This research study looks at the DNA of people with severe infections and injuries. Infectious diseases and severe injuries affect millions of people around the world every year. Most cases are mild, but some people become very unwell and are admitted to intensive care. Our genes (DNA) can determine how much critical illness affects us.
This study is running in our intensive care unit and aims to find the genes that cause some people to be more unwell. If we do, we may be able to develop better treatments for patients in the future. Currently this study will focus on patients admitted to ITU with COVID-19 to help understand if their genetics influence their response to this infection.
Data at the RUH is also being collected in specific disease areas, these include Oncology, Maternity, Dermatology and Rheumatology.
This study aims to collect information about COVID-19 in pregnant women and babies from around the world. The research aims to find out more about the effect of COVID-19 on early pregnancy, the growth of babies, early delivery and possible infection of babies. The information collated as part of this research will be shared with healthcare professionals internationally, allowing them to improve the care they give.
Neonatal Complications of Coronavirus
The study aims to find out how many babies develop coronavirus infection in the first month after birth and how many babies born to women with coronavirus need neonatal care. It aims to describe which babies develop COVID-19 infection and what symptoms or signs they have and how COVID-19 in babies is identified and treated.
Information on studies
Data at the RUH is also being collected in specific disease areas, these include Oncology, Maternity, Dermatology and Rheumatology.
Information about chest imaging is also being collected to support the development of a diagnostic tool.
With this ever evolving situation the research team at the RUH is preparing to take on more studies to help the national and international effort to understand and treat COVID-19.




